

Document Type : Review
Authors
1 Department of Microbial Biotechnology, Lorestan University of Medical Sciences, Azna Health Network, Lorestan, Iran
2 Department of Biology, Science Faculty, Shahrekord Islamic Azad University, Shahrekord, Iran
Abstract
Highlights
Keywords
Main Subjects
Introduction
The cause of cancer
All cancer cells continue to grow, divide, and divide again, forming new abnormal cells (1). Generally, cancer cells are produced from normal cells due to DNA damage. Often when DNA is damaged, the body can repair it; unfortunately, in cancer cells, the damaged DNA cannot be repaired (2). People can also inherit damaged DNA from their parents and a predisposition to cancer (3). Cancer cells have characteristics that can distinguish them from normal cells. One of the essential differences between these cells is their non-specialized function compared to normal cells (Figure 1) (4). This means that while normal cells mature into different types of cells with specific procedures, cancer cells do not have this ability. It can be seen that cancer cells have some disorder in the path of natural growth and development, or in other words, they have a developmental disorder (5). Sometimes tumors are benign and do not grow, but if the tumor cells grow, divide, destroy the surrounding normal cells, and invade other body parts, it is considered malignant. The most significant danger of malignant tumors is their ability to attack healthy tissues and spread in the body, and this is cancer metastasis (6) & they grow by increasing the production of several types of factors, as a result of which the formation of local blood vessels begins (7). Cancer cells that can hide these changes from the immune system are freed from suppression by the immune system and, in the next stage, suppress the immune response, which is called the escape stage. In the escape stage, the microenvironment around the tumor accumulates. Cells and molecules suppress the immune system (8).

Figure 1. Some Important causes of cancer (4)
Prostate anatomy
The prostate gland in the male reproductive system produces a fluid that helps transport sperm (Figure 2) (9). The consensus is that benign prostatic hyperplasia occurs in TZ, but prostatitis and cancer mainly occur in PZ. The CZ region of the prostate is rarely malignant (Figure 3) (10).

Figure 2. Prostate anatomy (9)
The onset of prostate disorders is primarily related to aging, which increases the sensitivity of the prostate tissue to damage or infection and leads to an increase in the inflammatory response (11).

Figure 3. Position of Prostate (10)
Prostate Cancer
The standard treatment options introduced for prostate cancer include surgery, radiation therapy, and chemotherapy, which often use these three methods together. In conventional patients, almost two-thirds of cancer patients are treated by radiation therapy (Figure 4) (12,13).
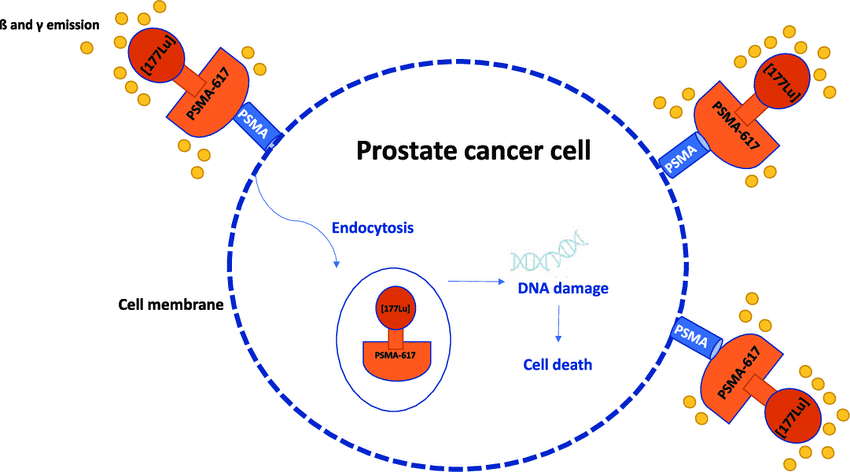
Figure 4. Prostate Cancer Cell (13)
Inflammation and prostate cancer, the role of PIA
Histologically, most prostate lesions that include chronic or acute inflammatory infiltration are related to the atrophic epithelium and focal epithelial atrophy (14). The epithelium appears atrophic in almost all cases, indicating that these areas can be considered PIA in the transition zone. On the other hand, it has been shown that molecular pathways are also changed in PIA lesions (15). For example, the protein products of three prostate tumor suppressor genes, namely 27 NKX3 and CDKN1B, which encodes p27, and phosphatase and its homolog PET18 and tensin 23 are less expressed in focal atrophy lesions. These genes are highly expressed in normal prostate epithelium and are often absent or reduced in PIN and prostate cancer (16). There is inflammation in radical specimens in prostate biopsies, often prostatectomy and tissues removed in treating BPH. Also, inflammatory cell infiltrates are usually found in and around the atrophy focus, characterized by an increased proliferative index (17). These foci are called PIA and may be precursors of early prostate cancer or represent a favorable environment for cancer development (18).
Lutetium 177
Luteium-177 has been chosen as a therapeutic radionuclide because of its favorable physical properties. Lu177 emits beta with a maximum energy of 0.498 megaelectron volts and gamma rays with 208 Kev and a frequency of 11%, and an energy of Kev 113 and a frequency of 6%. Other gamma rays with energy Kev 249 and Kev 321 are emitted with less intensity (19). The emission of gamma rays allows the conditions to be prepared for internal imaging and, thus, the collection of information related to radiation absorption in the tumor and dosimetry. Lu177 has a relatively long physical half-life of about 162 hours (equivalent to 6 or 7 days) (Figure 5) (20).
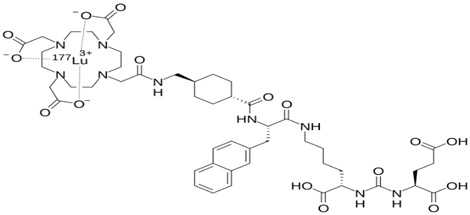
Figure 5. Structure of Lu177 (20)
Treatment method with 177LU PSMA radiopharmaceutical
Radioligand therapy is a type of radiation therapy that targets diffuse beta particles and a specific receptor in cancer cells to deliver targeted radiation. Prostate-specific membrane antigen is a receptor on the surface of prostate cancer cells. Lu_Prostate-specific membrane antigen (PSMA) 177 radioactive substance shown with PSMA peptides is used to treat prostate cancer (Figure 6) (20).

Figure 6. Treatment method with 177LU PSMA radiopharmaceutical (20)
Lutetium treatment
Lutetium or Lutetim is a treatment combined with identifiers and diagnostic science; This approach is also known as "Theranostics." Lutetium treatment is an option for advanced cancer treatment and is recommended for patients with advanced and metastasized prostate cancer (Figure 7) (21).

Figure 7. Lutetium treatment (21)
Effect of lutetium treatment on prostate cancer
PSMA is a protein expressed in the membrane of prostate cells and is believed to have multiple cellular functions. The prostate's epithelium, or lining tissue, naturally produces deficient levels of PSMA, while cancerous prostate tumors contain high levels of it—often 1,000 times higher than a normal prostate cell. Lutetium-177 effectively damages cancer cells by irradiating beta rays and destroying them over time. From where the lutetium molecule connects to PSMA, this method will become an exact treatment by targeting PSMA molecules (20,22).
The radiation used in this treatment is designed to destroy cancer cells only. Using theranostics, the medicine is more personalized than before so that the exact location of cancer & is destroyed, and the whole body will never be exposed to radiation (Figure 8) (23).

Figure 8. PSMA-Radio Surgery (Secure Treatment with Lutetium) (23)
Methods
This study is descriptive, and according to the implementation method, it is considered a systematic or systematic review. A systematic review is a structured literature review that focuses on one question and attempts to provide an answer by analyzing all available valid evidence. This type of review is done by searching for sources, using predetermined entry and exit criteria, critically evaluating evidence, and extracting and generating data from evidence and generated from them. Today, review articles are increasing uncontrollably as developing countries try to improve their position in domestic and international research rankings.
The review of past research is done in different ways. One of the most well-known is a systematic review, a type of secondary study and analysis of previous studies. In other words, a systematic review is a structured search based on predetermined rules and criteria. The statistical population of this systematic study includes all research and scientific articles, patents, and theses from quantitative prostate cancer studies and lutetium radiopharmaceuticals published between 2000 and 2022.
The criteria for the inclusion of articles in the present review include
The exclusion criteria of the articles included in the present review
1-All studies that were presented in seminars or conferences.
2-Studies were conducted on animal samples.
3- All articles were in languages other than English or Farsi.
4-repeated studies outside the desired period were excluded from the present study—information gathering process.
In this systematic review, the articles published between 1380 and 1401, which in some way investigated prostate cancer and the treatment methods used, such as lutetium radiopharmaceutical, were studied and analyzed. The research was searched using reliable Persian and English databases such as ISID, Google Scholar،, Science Direct, PubMed, World Health Organization website, and searching by Persian and English keywords such as prostate cancer, lutetium, and radiopharmaceutical. Finally, 150 articles from 300 articles entered the study phase for review.
Review of studies done
In 2019, Sarnelli et al., with a study entitled 177Lu PSMA dosimetry after mannitol and glutamate injection, evaluated the dose of the internal organs of the body after the administration of the glutamate drug and investigated the effect of this drug on reducing or increasing the dose of the internal organs of the body. They put the protection laws of Italy as the basis of their work. In their study, nine patients were selected as samples, and using whole body images at different times, the average dose absorbed in the parotid glands was estimated at 0.48 mGy / MBq and 0.70 mGy / MBq for the kidneys. According to his study, this drug can partially reduce the absorbed dose in the parotid glands (24).
In the studies of Mayer et al., (2018), 25 patients with prostate cancer were investigated in the first treatment cycle. The purpose of their study was to analyze the dose of the patients at discharge, as well as the dose of the general public and the patient's family members, and the dose for the patient was calculated at a distance of 2 meters. Based on those studies, the laws of Australia were implemented, and the dosage limit of msv1 for family members and msv3 for the patient's caregivers was determined. The average radiation exposure of the patient's companions in his study was recorded as 202.3 ± 42.7 μsv (25).
Kabasakal et al., (2017) examined the patients treated with PSMA drug in terms of drug stability during drug distribution in the body and dosimetry of internal body organs (kidneys, parotid glands, bone marrow) and their study. Seven patients were selected in the first treatment cycle, and the amount of absorbed dose was calculated for parotid glands at 1.90±1.19 Gy / GBq and kidneys at 0.82±0.25 Gy / GBq.In the continuation of their studies, they calculated the amount of internal organs of 7 patients using PET / CT images only in the first stage of treatment. Blood samples were used at different hours after the injection to obtain the absorbed dose in the bone marrow, and the highest amount for Parotid glands was estimated to be 1.17±0.31 mGy (26).
Demir et al., (2016) examined 23 patients undergoing prostate cancer treatment in terms of the protection laws of Turkey and measured their total body dose at 0, 25, 1, and 2 meters at different times after injection. Moreover, it compared it with the set protection criterion sv/hμ 30 and declared that according to the protection laws of Turkey, this treatment could be provided on an outpatient basis. The dose received by the patient's companions in this research is estimated at svμ 260 svμ 120 (27).
Sundström et al., (2010) in their research calculated the absorbed dose during lutetium-177 in the first stage of treatment for kidney and bone marrow using whole body images obtained at different times during the treatment which 200 patients under The treatment with GBq 7.4 of the drug was investigated and the values obtained for the bone marrow were calculated in the range of 0.05 Gy and 2 Gy10 for the kidneys. In their study, Turner and his colleagues (2012) examined the physical conditions and discharge of patients treated with different radionuclide drugs, including iodine 131 and lutetium 177, in terms of the physical and biological conditions of each drug. The type of radiation and treatment conditions were stated (28).
Rahbar et al., (2018) studied PSMA on 56 prostate cancer patients treated with radiopharmaceutical 177 Lu. According to their report, while the average absorption of PSMA in the salivary glands was higher than the dose absorbed by the kidneys, only two patients (4%) experienced xerostomia (dry mouth) transiently after 3 and 4 courses (29).
Conclusion and Recommendations
The number of cancer patients is increasing yearly, a medical problem. Cancer is a disease that is not produced by physical injuries. From the time the first cell mutates and turns into a cancer cell, it takes about 5 to 7 years until a cancerous mass is formed. Therefore, in any disease, the first protocol is to prevent it, and if the disease occurs, it is to diagnose it early and use the best treatment method. The good news is that many cancers are preventable. Some of these diseases can be cured if they are diagnosed on time. Half of the people who get cancer have a good chance of surviving. Many causes of cancer and measures aimed at reducing their risks are known. Currently, because many cancers are diagnosed early, the patient can be kept alive to a large extent and continue his life. This is possible by performing self-examination and screening. Prevention of cancer is not limited to a certain age; one should try to improve one's lifestyle and society with gradual changes in the way of life (30).
Although inflammation and prostate cancer have been researched for many years, no infectious agent that alone has a definitive relationship with the development of prostate cancer has been proven. Despite this, there is evidence for the role of some prostate infections in the carcinogenesis process by inducing long-term chronic inflammation. In general, a pathogen that has not been identified so far may be seen in the prostate of cancer patients, or it is possible that the chronic inflammation observed in the prostate of cancer patients started years before the development of cancer and is not related to the high prevalence of the infectious agent. This hypothesis is consistent with recent studies that describe prostate infections due to Propionibacterium acnes in rodent models. Several studies have emphasized the importance of previous exposure to sexually transmitted microbial agents and a history of prostatic disease in the natural history of prostate cancer. More future research should be done, along with the investigation of serological markers of infectious agents or markers predicting chronic inflammation to clarify the possible path of chronic and recurrent infection in the etiology of prostate cancer.
Declarations
Author’s contributions
All authors contributed equally.
Acknowledgments
Special thanks to the Department of Microbial Biotechnology, Lorestan University of Medical Sciences, Azna Health Network, Lorestan, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding
No funding.
Ethical statement
Not applicable.
Data Availability
No additional data are available for this article.
Abbreviations
PIA Proliferative inflammatory atrophy
PSMA Prostate-specific membrane antigen